Understanding HOCl PH: A Comprehensive Guide To Its Importance And Applications
HOCl pH plays a crucial role in various industries, from healthcare to water treatment, yet many people remain unaware of its significance. Hypochlorous acid (HOCl) is a powerful yet gentle disinfectant that has gained increasing attention due to its effectiveness and safety. Whether you're a professional in the sanitation industry or simply interested in maintaining a clean environment, understanding the pH levels of HOCl is essential. This article will explore the science behind HOCl pH, its applications, and how it can be optimized for different purposes.
When discussing HOCl, it's important to understand that its effectiveness is heavily influenced by its pH level. The pH scale measures how acidic or basic a solution is, and for HOCl, maintaining the right pH is critical for maximizing its disinfectant properties. In this article, we will delve into the factors that affect HOCl pH, its benefits, and how it compares to other disinfectants. By the end, you'll have a thorough understanding of why HOCl pH is a key consideration in sanitation and hygiene.
As we navigate through this topic, we will also address common misconceptions about HOCl and its pH levels. Many people mistakenly believe that higher concentrations of disinfectants are always better, but this is not the case with HOCl. The pH level directly impacts its stability and efficacy, making it a delicate balance that requires careful management. Let's dive deeper into the world of HOCl pH and uncover its potential to revolutionize cleaning and disinfection practices.
Read also:Deion Sanders Career Earnings A Comprehensive Look At His Financial Success
Table of Contents
- What is HOCl?
- The Importance of pH in HOCl Solutions
- Factors Affecting HOCl pH Levels
- Applications of HOCl in Various Industries
- Benefits of Using HOCl with Optimal pH
- HOCl vs. Other Disinfectants: A pH Perspective
- How to Maintain the Right pH for HOCl
- Safety Considerations When Handling HOCl
- Common Misconceptions About HOCl pH
- Conclusion and Call to Action
What is HOCl?
Hypochlorous acid (HOCl) is a weak acid that forms when chlorine dissolves in water. It is a naturally occurring compound produced by the human immune system to fight infections. HOCl is widely recognized for its powerful antimicrobial properties, making it a popular choice for disinfection and sanitization. Its effectiveness lies in its ability to penetrate microbial cell walls and disrupt their internal structures, leading to rapid inactivation.
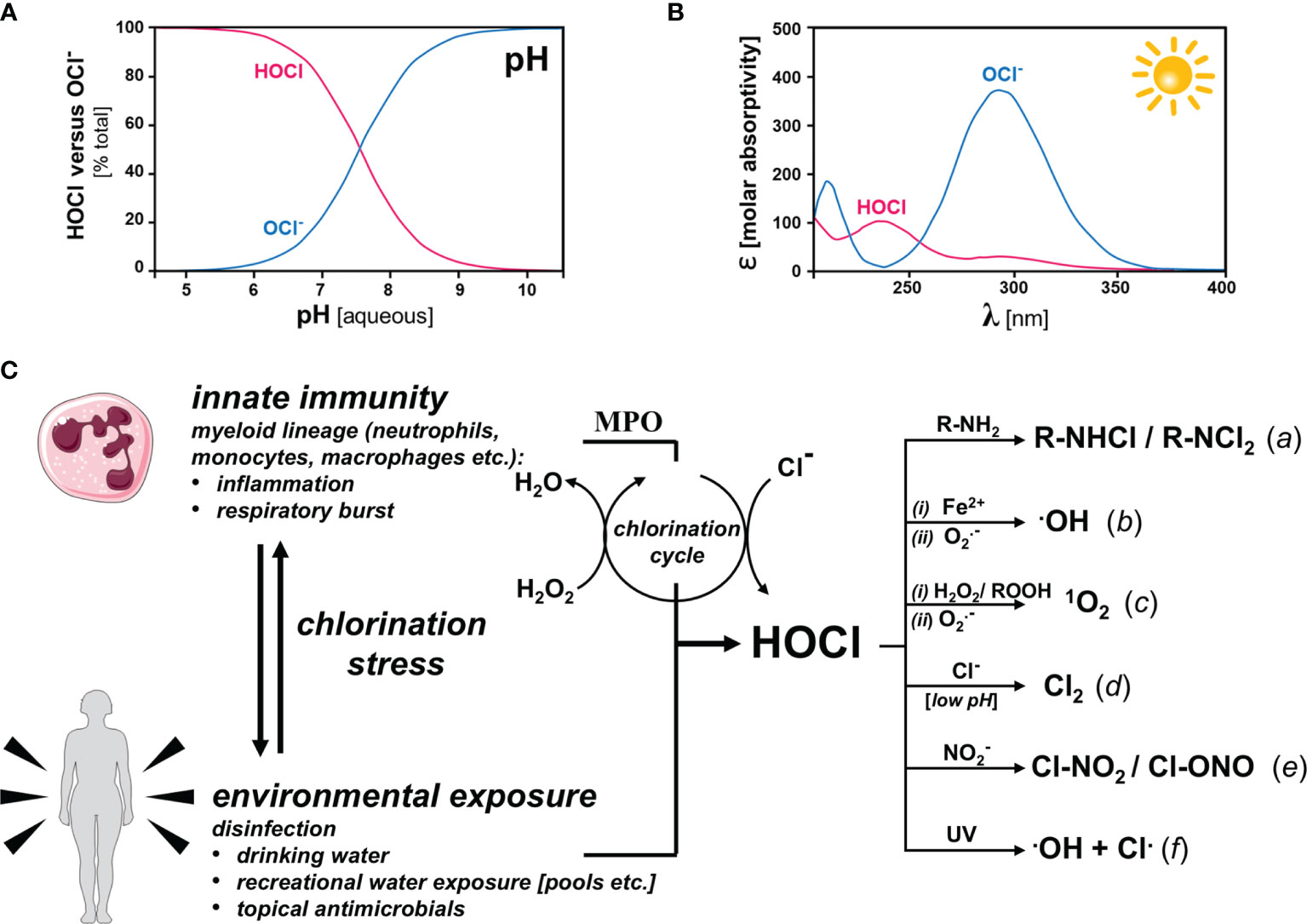
One of the key characteristics of HOCl is its pH sensitivity. The molecule exists in equilibrium with its conjugate base, hypochlorite ion (OCl⁻), depending on the pH of the solution. At lower pH levels, HOCl is the dominant form, while at higher pH levels, OCl⁻ becomes more prevalent. This balance is crucial because HOCl is far more effective as a disinfectant compared to OCl⁻.
HOCl is used in a variety of applications, including healthcare, food processing, and water treatment. Its ability to disinfect without leaving harmful residues makes it an environmentally friendly option. Understanding the science behind HOCl pH is essential for maximizing its benefits in these industries.
The Importance of pH in HOCl Solutions
The pH level of an HOCl solution is a critical factor that determines its effectiveness as a disinfectant. HOCl is most potent in slightly acidic to neutral pH ranges, typically between 5 and 7. Within this range, the majority of the chlorine present is in the form of HOCl, which is significantly more effective at killing pathogens compared to its counterpart, OCl⁻.
When the pH rises above 7, the proportion of OCl⁻ increases, reducing the solution's disinfectant capabilities. Conversely, extremely low pH levels can cause HOCl to become unstable, leading to the release of chlorine gas, which poses health risks. Therefore, maintaining the optimal pH range is vital for ensuring both the safety and efficacy of HOCl solutions.
Understanding the importance of pH in HOCl solutions is not only crucial for professionals in sanitation and healthcare but also for consumers using HOCl-based products. By optimizing the pH, users can achieve better results while minimizing potential risks.
Read also:Steve Mcqueen The Timeless Legend Of Hollywoods King Of Cool
Why pH Matters in Disinfection
- HOCl is 80-100 times more effective than OCl⁻ at killing pathogens.
- Optimal pH ensures the stability and longevity of HOCl solutions.
- Incorrect pH levels can lead to reduced effectiveness or safety hazards.
Factors Affecting HOCl pH Levels
Several factors can influence the pH of HOCl solutions, and understanding these variables is essential for maintaining their effectiveness. The source of the HOCl, the method of production, and environmental conditions all play a role in determining the final pH of the solution.
One of the primary factors is the production method. HOCl can be generated through electrolysis of a saltwater solution, and the pH of the resulting solution depends on the electrolysis parameters. Adjusting the current and electrolyte concentration can help control the pH. Additionally, the quality of the water used in the process can impact the pH, as impurities may alter the chemical balance.
Environmental factors such as temperature and exposure to air can also affect HOCl pH. For example, higher temperatures can cause the solution to become more acidic, while exposure to carbon dioxide in the air can lead to a slight increase in pH. Proper storage and handling are therefore crucial for preserving the desired pH level.
Common Production Methods and Their Impact on pH
- Electrolysis: Allows precise control over pH through parameter adjustments.
- Chemical synthesis: May result in higher pH levels without proper buffering.
- Commercial products: Often formulated with stabilizers to maintain pH.
Applications of HOCl in Various Industries
HOCl is widely used across multiple industries due to its versatility and effectiveness. In healthcare, it is employed for wound care, surface disinfection, and sterilization of medical equipment. Its non-toxic nature makes it safe for use in sensitive environments, such as hospitals and clinics.
In the food industry, HOCl is utilized for sanitizing surfaces, equipment, and even produce. It is approved by regulatory agencies such as the FDA and EPA for use in food processing due to its ability to eliminate pathogens without leaving harmful residues. This makes it an ideal choice for ensuring food safety.
Water treatment is another significant application of HOCl. It is used to disinfect drinking water, swimming pools, and wastewater. Its ability to kill bacteria, viruses, and other microorganisms while being environmentally friendly has made it a preferred alternative to traditional chlorine-based treatments.
Benefits of HOCl in Specific Industries
- Healthcare: Reduces the risk of hospital-acquired infections.
- Food processing: Ensures compliance with safety regulations.
- Water treatment: Provides safe and effective disinfection.
Benefits of Using HOCl with Optimal pH
Using HOCl with the correct pH offers numerous advantages, both in terms of performance and safety. One of the primary benefits is its enhanced disinfectant properties. When the pH is within the optimal range, HOCl is highly effective at eliminating a wide range of pathogens, including bacteria, viruses, and fungi.
Another significant advantage is its safety profile. HOCl solutions with the right pH are non-toxic, non-irritating, and safe for use on skin and surfaces. This makes them suitable for applications where traditional disinfectants might pose risks, such as in households with children or pets.
Additionally, HOCl is environmentally friendly. Unlike many chemical disinfectants, it breaks down into harmless byproducts, reducing its environmental impact. This aligns with the growing demand for sustainable and eco-conscious solutions in various industries.
Why Optimal pH Enhances HOCl Benefits
- Maximizes pathogen-killing efficiency.
- Ensures safety for users and the environment.
- Extends the shelf life of HOCl solutions.
HOCl vs. Other Disinfectants: A pH Perspective
When comparing HOCl to other disinfectants, its pH sensitivity sets it apart. Traditional chlorine-based disinfectants, such as bleach, often have higher pH levels, which can reduce their effectiveness and increase the risk of skin irritation. HOCl, on the other hand, operates best in a slightly acidic to neutral pH range, making it both more potent and safer.
Alcohol-based disinfectants, while effective, can be drying and irritating to the skin. HOCl solutions with optimal pH levels provide a gentler alternative without compromising on efficacy. This makes them particularly suitable for frequent use in healthcare and household settings.
Quaternary ammonium compounds (quats) are another common disinfectant, but they can leave residues that may contribute to antimicrobial resistance. HOCl, with its pH-optimized formulation, avoids this issue by breaking down into harmless components after use.
Key Differences Between HOCl and Other Disinfectants
- HOCl is more effective at lower concentrations compared to bleach.
- Less irritating to skin and surfaces than alcohol-based solutions.
- Environmentally friendly compared to quats and other chemical disinfectants.
How to Maintain the Right pH for HOCl
Maintaining the correct pH for HOCl solutions requires careful attention to production, storage, and usage. One of the most effective ways to ensure optimal pH is to use a pH meter or test strips to monitor the solution regularly. This allows for adjustments to be made as needed to keep the pH within the desired range.
Buffering agents can also be added to stabilize the pH of HOCl solutions. These agents help resist changes in pH when the solution is exposed to environmental factors or contaminants. However, it's important to choose buffering agents that do not interfere with the disinfectant properties of HOCl.
Proper storage is another critical factor. HOCl solutions should be kept in airtight containers to prevent exposure to air, which can alter the pH. Additionally, storing the solution in a cool, dark place can help maintain its stability and effectiveness over time.
Tips for Maintaining HOCl pH
- Regularly test the pH using reliable tools.
- Use buffering agents to stabilize the solution.
- Store in airtight containers away from light and heat.
Safety Considerations When Handling HOCl
While HOCl is generally safe to use, certain precautions should be taken to ensure its safe handling and application. One of the primary safety considerations is maintaining the correct pH. Solutions with excessively low pH levels can release chlorine gas, which is hazardous to health. Therefore, it's crucial to monitor and adjust the pH as needed.
Personal protective equipment (PPE) should be worn when handling concentrated HOCl solutions. This includes gloves and goggles to protect against potential splashes or spills. Additionally, users should avoid mixing HOCl with other chemicals, as this can lead to dangerous reactions.
Proper ventilation is also important when using HOCl in enclosed spaces. While HOCl is less volatile than bleach, ensuring good airflow can help prevent the buildup of any fumes that may be released during use.
Safety Tips for Using HOCl
- Always wear appropriate PPE when handling concentrated solutions.
- Avoid mixing HOCl with other chemicals to prevent reactions.
- Ensure good ventilation in areas where HOCl is used.
Common Misconceptions About HOCl pH
Despite its growing popularity, there are several misconceptions about HOCl and its pH levels. One common misconception is that higher concentrations of HOCl are always better. In reality, the pH level is a more critical factor than concentration when it comes to effectiveness.
Another misconception is that HOCl solutions are inherently unstable. While it's true that pH imbalances can affect stability, properly formulated and stored HOCl solutions can remain effective for extended periods. Using buffering agents and airtight containers can help mitigate stability issues.
Finally, some people believe that HOCl is only suitable for industrial applications. However, its safety and versatility make it an excellent choice for household use as well. By understanding and addressing these misconceptions, users can make more informed decisions about incorporating HOCl into their cleaning
Horseshoe Symbolism: Unveiling Its Mystical And Cultural Significance
American Museum Of Natural History Free: Your Ultimate Guide To Exploring Without Spending A Dime
Exploring Castlemont High School: A Beacon Of Education And Community

Why pH Matters A Look Into HOCl Formulations BIHOCL Blog
Frontiers Hypochlorous Acid From Innate Immune Factor and